Formidable Tips About How To Build A Bomb Calorimeter

The dewar stops heat from.
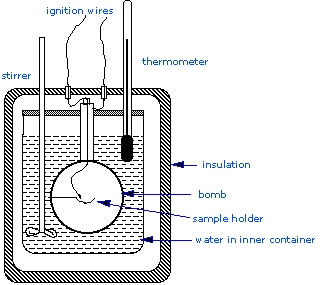
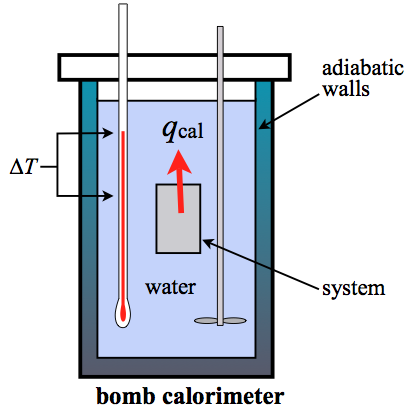
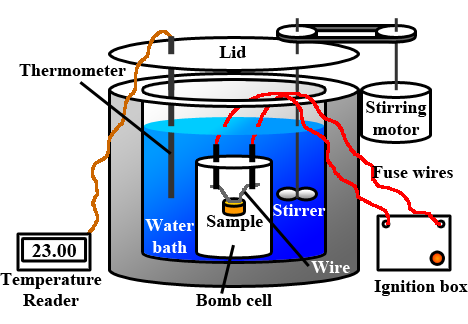
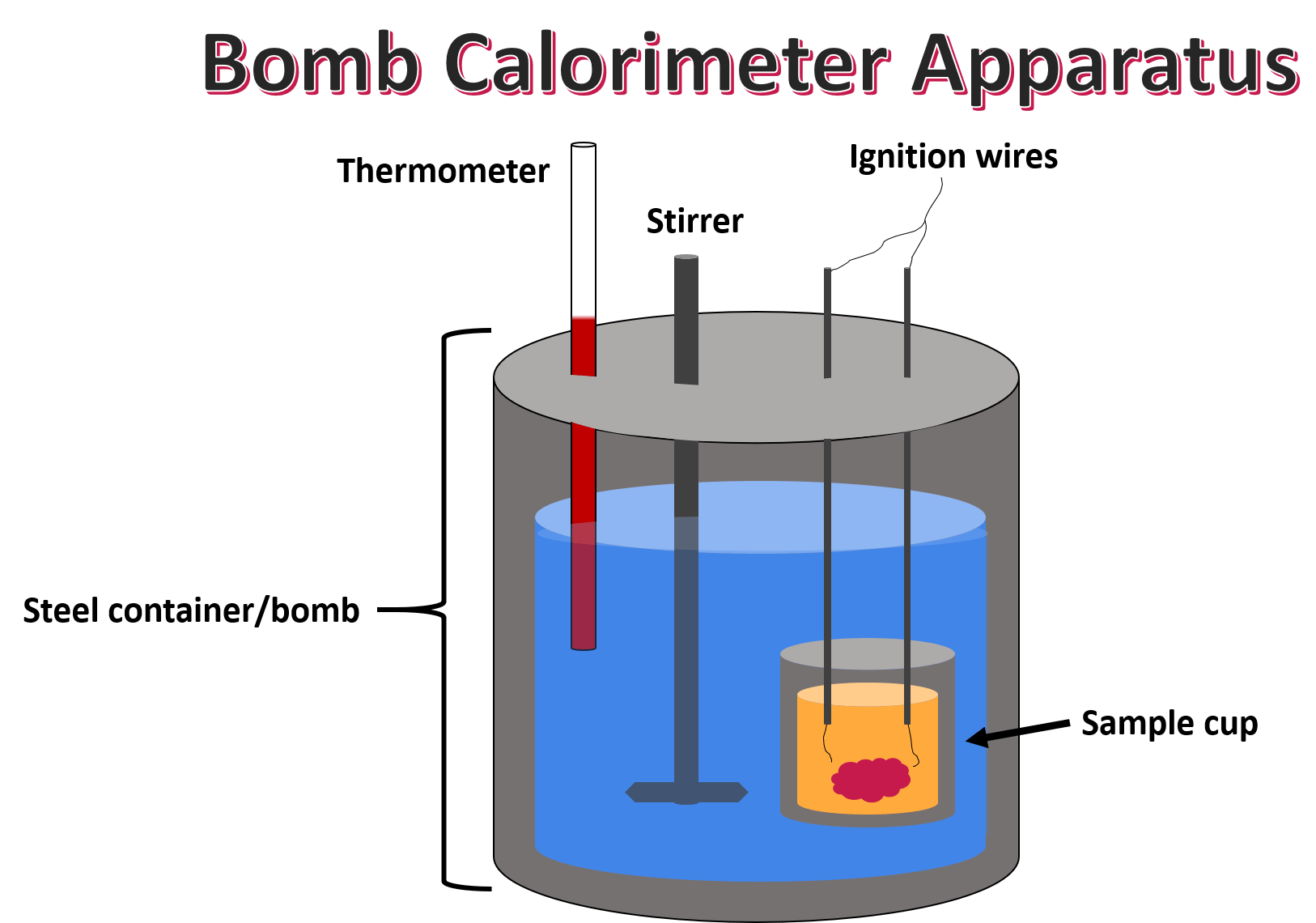
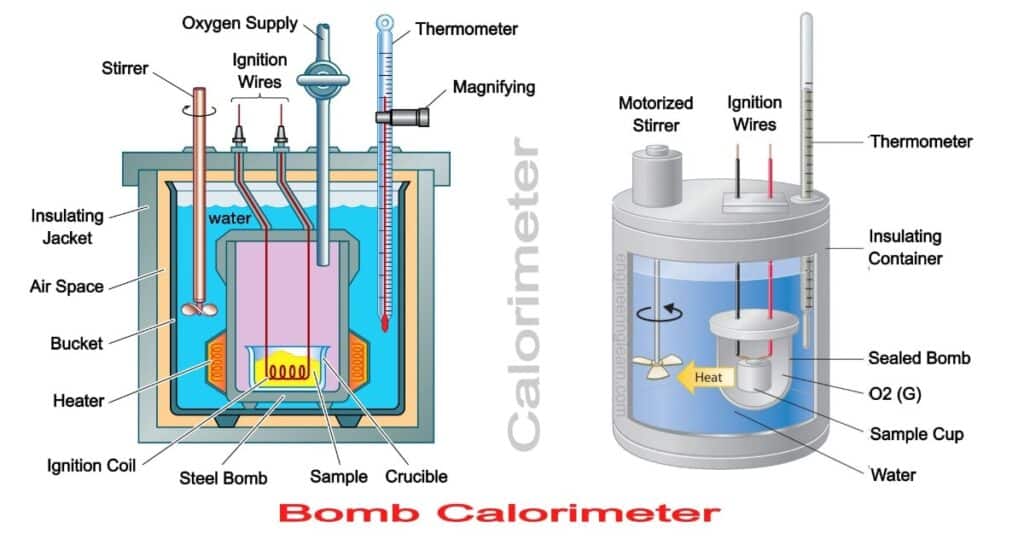
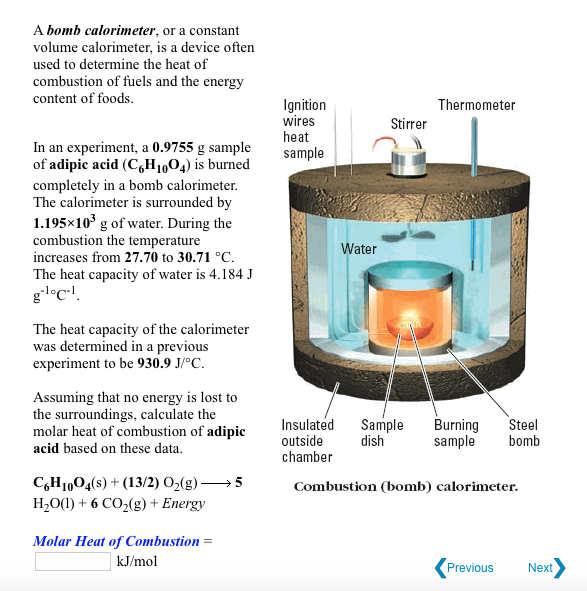
How to build a bomb calorimeter. Bomb calorimetry is used to determine the heat of combustion of a gas, liquid, or solid. The bomb calorimeter consists of pressurized oxygen “bomb” (30 bar), which houses the fuel. The bomb has a fixed mass and specific heat.
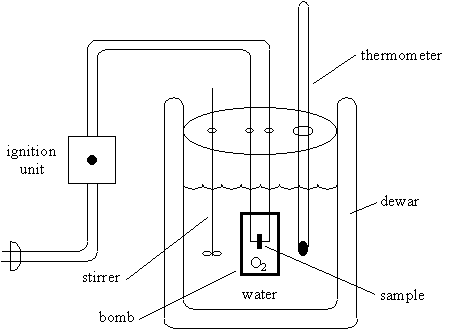
Charge oxygen in the bomb until the pressure reach 3.0mpa. A 10 cm fuse wire connected to two electrodes is kept in contact with the fuel inside the bomb. Put oxygen bomb in and turn on the calorimeter, burn the sample.
The bomb calorimeter consists of pressurized oxygen “bomb” (30 bar), which houses the fuel. Rate this post contents show 1 determine the required components to construct a calorimeter 2 constructing the calorimeter 3 utilizing the calorimeter 4 calculating 5 how to build a. We prepare the sample in an oxygen.
The mass of the bomb multiplied by its specific heat. Four essential parts are required in any bomb calorimeter: Put the pail in the calorimeter.
A 10 cm fuse wire connected to two electrodes is kept in contact with the fuel inside the bomb. Connect the ignition wires to the terminals on the bomb head. This video shows how a bomb calorimeter can be set up and operated.
Carefully put the bomb into the pail. A bomb calorimeter is an instrument used to measure the amount of heat generated in the combustion of a solid or liquid substance, operating at constant volume. The sample, oxygen, the stainless steel bomb, and water make up the bomb calorimeter's main components.
Put this crucible in oxygen bomb and seal it. Combustion is an exothermic reaction with oxygen.
/GettyImages-141482586-566245ac3df78ce16197a1fd.jpg)